FoMoCo
Member
- Joined
- Apr 4, 2008
- Messages
- 118
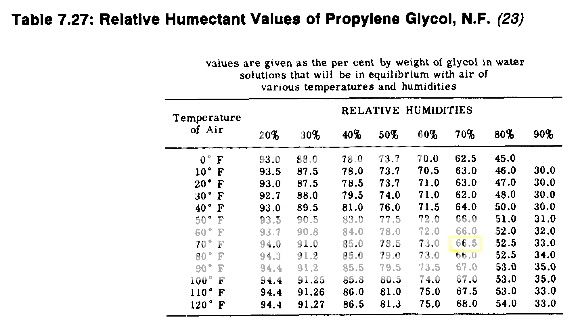
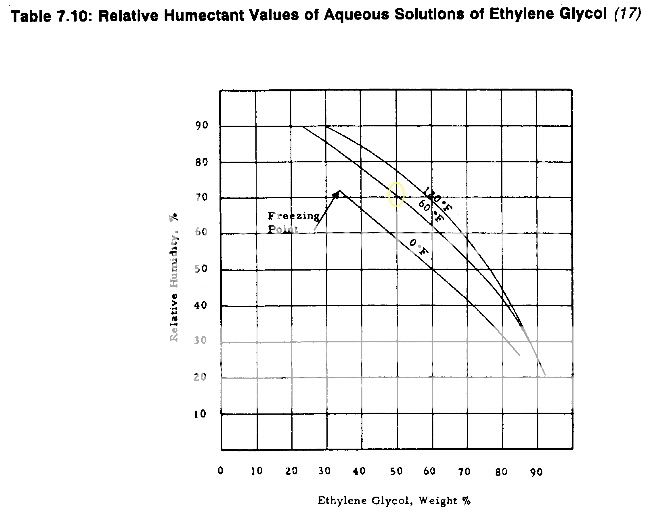
I have searched for information on using PG to maintain a RH level of 65% with little success. I have found differing opinions on the feasibility of this but without any confirmation either way. So I thought I would run a brief test to investigate it for myself. I prepared PG / distilled water solutions with concentrations of 80/20, 67/33, and 50/50. I applied the solution to oasis foam and sealed them in a Tupperware container with a calibrated hygrometer. I am not a scientist, but I did try to control the variables in testing such that the only significant change was the PG / water concentration.
Results:
50/50 = 70% +/-1 - baseline
67/33 = 65% +/-1
80/20 = 60% +/-1
Questions:
Did I miss the thread that already covered this?
Anyone else using PG to maintain 65%?
Are these experimental results expected theoretically?
Before you reply with "Get 65% beads", I am aware of the bead products and may try them in the future. However, I had PG and distilled water on hand and was curious if it would maintain 65% RH.
Results:
50/50 = 70% +/-1 - baseline
67/33 = 65% +/-1
80/20 = 60% +/-1
Questions:
Did I miss the thread that already covered this?
Anyone else using PG to maintain 65%?
Are these experimental results expected theoretically?
Before you reply with "Get 65% beads", I am aware of the bead products and may try them in the future. However, I had PG and distilled water on hand and was curious if it would maintain 65% RH.