preembargo
Sleeping not so peacefully
-RELATIVE HUMIDITY-
SATURATED SALT SOLUTION TEST
(For Wilkeyites)
Short description
This validation method measures relative humidity in a range of 0 - 100% using saturated solutions of various salts at various temperatures. This method relies on the principle that the partial vapor pressure of water above a saturated salt solution in equilibrium with its surroundings is constant at known temperatures.
In other words, by placing a saturated salt solution in a sealed container, the relative humidity of the air column above the solution can be precisely measured at specific temperatures. A list of saturated salts and their humidity values at different temperatures is shown in the following table.
Note that these temperature values are useful for the purposes of precise calibrations but the variations in RH due to temperature are negligible and therefore are of little consequence regarding RH values within your humidor.
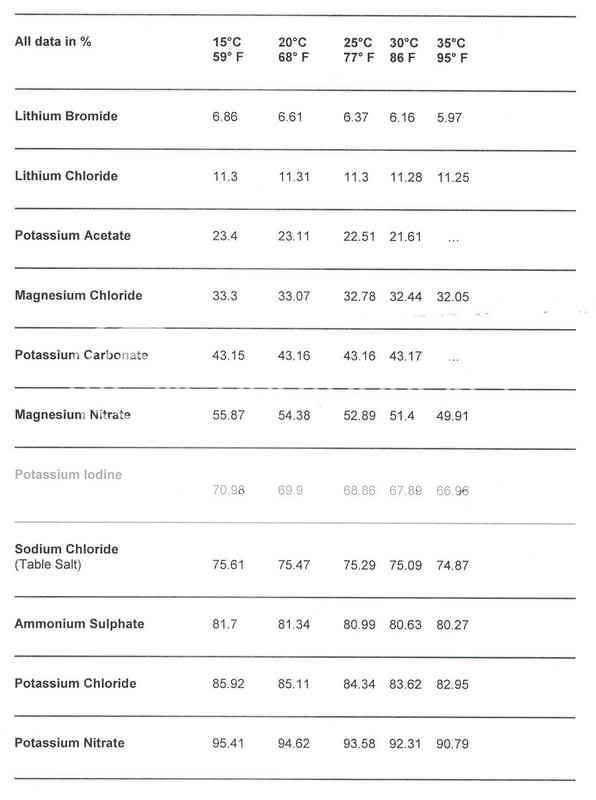
(By the way, if you have Potassium Iodine lying around, you clearly have bigger concerns than calibrating your hygrometer. Just make sure your Geiger Counter is calibrated)
Calibration procedure:
- Place about 1 inch of saturated salt solution in an open container that is approximately 4 inches high. The 3 inches above the solution will contain air with a predictable humidity value.
- Place the container with the saturated salt solution in a sealable container. The hygrometer must be suspended in the saturated air column above the salt solution. Do not get the salt or salt solution on the hygrometer.
- Seal the container. Allow 6 to 8 hours for the air inside the hygrometer to reach equilibration.
-Compare your readings to the values in the above table.
- If you’re obsessive compulsive, repeat the above procedure using various saturated salt solutions from the above table.
The problem with using salt solutions for measuring relative humidity
It is known and accepted that relative humidity is one of the physical quantities most difficult to calibrate. The primary difficulty is to generate humidity with a high degree of stability for the purpose of precise calibration. All classical methods require either temperature stability and uniformity or accurate measurement of the temperature.
The water vapor concentration, and therefore the relative humidity over a salt solution is less than that over pure water. This is because water is present in both the gas and the liquid phase, whereas the salt molecules are only present in the liquid. During this process the saturated solution begins to absorb water from the air. This dilutes the solution at the surface. The less dense surface solution is gravitationally stable and does not mix with the bulk of saturated solution, so the RH continually drifts upwards from the value for the saturated solution towards 100%. If the room temperature is unstable the salt solution behaves quite unpredictably, causing considerable errors when such solutions are used to calibrate your hygrometer.
The situation can be improved by heaping up the solid salt in a mound, with just enough water to dampen it. The solution formed by water absorption from the air will roll down the outside of the mound, continually renewing the crystal surface enabling it to grab more water from the air. This process is known as convective mixing. Convective mixing can also be achieved by enclosing the saturated solution in a polymer sachet that is permeable to water molecules but not to charged ions. Thin silicone sheeting works well for this purpose but then the test has become so complicated that it is better to use another RH control method altogether.
This argument applies to all salt solutions, saturated or not. The reason for using saturated solutions, in contact with a heap of the solid crystals, is that the concentration theoretically remains constant even if water enters or leaves the solution from the air.
How To Use Saturated Salt Solutions For Calibrating Hygrometers
–English Version-
(For the rest of us)
The dry salt is placed in a mound about 1 inch deep in a small tray. Water is added to moisten the salt. Do not add more water than is needed to make the salt look damp. The tray is placed in an airtight bag or box. The hygrometer is then placed in the container with the damp salt. In about 6-8 hours your hygrometer should read 75%.
edited to add: Mostly plagiarized from various sources, I ain’t that book lerned.
________________________________________
SATURATED SALT SOLUTION TEST
(For Wilkeyites)
Short description
This validation method measures relative humidity in a range of 0 - 100% using saturated solutions of various salts at various temperatures. This method relies on the principle that the partial vapor pressure of water above a saturated salt solution in equilibrium with its surroundings is constant at known temperatures.
In other words, by placing a saturated salt solution in a sealed container, the relative humidity of the air column above the solution can be precisely measured at specific temperatures. A list of saturated salts and their humidity values at different temperatures is shown in the following table.
Note that these temperature values are useful for the purposes of precise calibrations but the variations in RH due to temperature are negligible and therefore are of little consequence regarding RH values within your humidor.

(By the way, if you have Potassium Iodine lying around, you clearly have bigger concerns than calibrating your hygrometer. Just make sure your Geiger Counter is calibrated)
Calibration procedure:
- Place about 1 inch of saturated salt solution in an open container that is approximately 4 inches high. The 3 inches above the solution will contain air with a predictable humidity value.
- Place the container with the saturated salt solution in a sealable container. The hygrometer must be suspended in the saturated air column above the salt solution. Do not get the salt or salt solution on the hygrometer.
- Seal the container. Allow 6 to 8 hours for the air inside the hygrometer to reach equilibration.
-Compare your readings to the values in the above table.
- If you’re obsessive compulsive, repeat the above procedure using various saturated salt solutions from the above table.
The problem with using salt solutions for measuring relative humidity
It is known and accepted that relative humidity is one of the physical quantities most difficult to calibrate. The primary difficulty is to generate humidity with a high degree of stability for the purpose of precise calibration. All classical methods require either temperature stability and uniformity or accurate measurement of the temperature.
The water vapor concentration, and therefore the relative humidity over a salt solution is less than that over pure water. This is because water is present in both the gas and the liquid phase, whereas the salt molecules are only present in the liquid. During this process the saturated solution begins to absorb water from the air. This dilutes the solution at the surface. The less dense surface solution is gravitationally stable and does not mix with the bulk of saturated solution, so the RH continually drifts upwards from the value for the saturated solution towards 100%. If the room temperature is unstable the salt solution behaves quite unpredictably, causing considerable errors when such solutions are used to calibrate your hygrometer.
The situation can be improved by heaping up the solid salt in a mound, with just enough water to dampen it. The solution formed by water absorption from the air will roll down the outside of the mound, continually renewing the crystal surface enabling it to grab more water from the air. This process is known as convective mixing. Convective mixing can also be achieved by enclosing the saturated solution in a polymer sachet that is permeable to water molecules but not to charged ions. Thin silicone sheeting works well for this purpose but then the test has become so complicated that it is better to use another RH control method altogether.
This argument applies to all salt solutions, saturated or not. The reason for using saturated solutions, in contact with a heap of the solid crystals, is that the concentration theoretically remains constant even if water enters or leaves the solution from the air.
How To Use Saturated Salt Solutions For Calibrating Hygrometers
–English Version-
(For the rest of us)
The dry salt is placed in a mound about 1 inch deep in a small tray. Water is added to moisten the salt. Do not add more water than is needed to make the salt look damp. The tray is placed in an airtight bag or box. The hygrometer is then placed in the container with the damp salt. In about 6-8 hours your hygrometer should read 75%.
edited to add: Mostly plagiarized from various sources, I ain’t that book lerned.
________________________________________



